See answer 1 Best Answer. Element 1 element 2 Forms ionic compound.

Hybridization Of Brf3 Bromine Trifluoride Molecules Understanding Chemical
IBr is best described as having polar covalent bonds.

. No it forms a. Chlorine and bromine j. Flourine is not a.
On the other hand sulfur S is located in period 3 group 16 of the periodic table and has six electrons on its outermost shell. Start studying Ionic Compounds. Learn vocabulary terms and more with flashcards games and other study tools.
Does magnesium and nitrogen form an ionic compound. Rubidium an alkali metal does not form compounds or ionic bonds with calcium an alkaline earth metal. Any metallic element will form an ionic compound with fluorine.
Sodium and potassium l. An ionic bond. The subscript for both calcium and oxygen is 1.
Up to 256 cash back Write a formula for the ionic compound that forms between each pair of elements. Potassium and sulfur k. Sodium and neon e.
Empirical formula of ionic compound iodine sulfur yes no chlorine calcium yes no П bromine sodium yes no 0 lithium sulfur yes no. Which of the following pairs of elements are likely to form an ionic compound. Kl Determine the chemical formula for the ionic compound that forms between rubidium and sulfur.
Sulfur and bromine will form a molecular compound because both sulfur and bromine are nonmetals. Magnesium and chlorine i. Nitrogen and fluorine g.
H3C-CH3 ethane has a non-polar bond C-C. Sulfur can form ionic compounds eg SO2 but Xenon is a noble gas and does not react to form compounds. Add electron dots and charges as necessary to show the reaction of potassium and bromine to form an ionic compound.
Magnesium Mg is located in period 3 group 2 of the periodic table and has two electrons on its outermost shell ie. Rubidium bromide - Wikipedia. Rubidium and bromine d.
Sodium and fluorine will form an ionic compound because sodium is a metal and fluorine is a nonmetal. The only truly non-polar bonds are between identical elements or compounds made with identical elements identically substituted. Answer 1 of 3.
It is a method to separate two compounds or mixtures depending upon. Can sulfur and bromine form an ionic compound. To determine the chemical formula that two nonmetal elements might form in a neutral molecular compound it s helpful to draw the partial Lewis.
Do bromine and sulfur form an ionic compond. Lithium and chlorine b. Science Chemistry QA Library do bromine and sulfur form an ionic compond.
Answer 1 of 2. Usually rubidium hydroxide or rubidium carbonate is reacted with hydrobromic acid. Therefore making the bond polar.
Dear Belinda The thermodynamic parameter which is of significance in this case is the Reduction Potential for molecular bromine which is 11 v vs NHE. As you know chemical reactivity is governed by an atoms desire to have a. Calcium and oxygen b.
Cesium and magnesium f. Potassium and sulfur make an ionic compound called potassium sulfide with the formula unit K2S. Oxygen and bromine c.
Want to see the full answer. Zinc and sulfur c. Therefore the ionic compound that forms between rubidium and bromine is.
Rubidium bromide is formed. The electronegativity difference between sulfur and bromine comes out to be 04 which means that the bond is polar covalent. Potassium and oxygen d.
Does sulfur and strontium form an ionic compound. Do bromine and sulfur form an ionic compond. The bromine will oxidize sulfur compounds in which the valence of sulfur is lower than six to sulfate.
There are many possible reactions. Helium and oxygen h. View the full answer.
In other words it is a strong oxidizing agent. This is because bromine is slightly more electronegative than sulfur so that the electron will be closer to bromine.
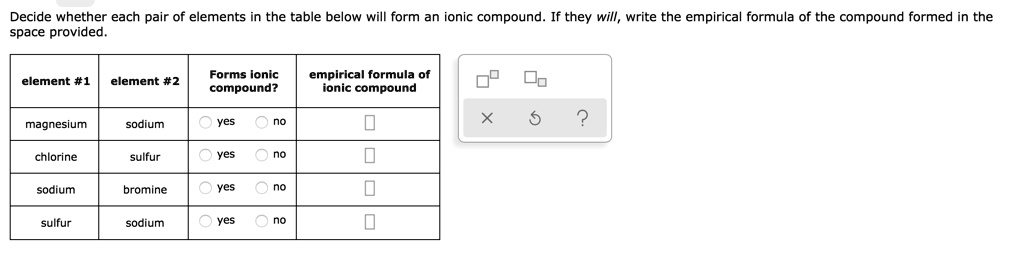
Solved Decide Whether Each Pair Of Elements In The Table Below Will Form An Ionic Compound If They Will Write The Empirical Formula Of The Compound Formed In The Space Provided Forms Ionic
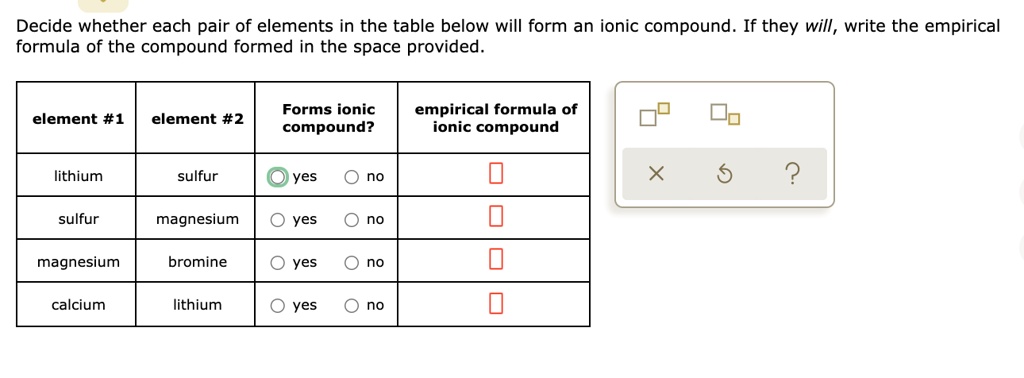
Solved Decide Whether Each Pair Of Elements In The Table Below Will Form An Ionic Compound If They Will Write The Empirical Formula Of The Compound Formed In The Space Provided Forms Ionic

Image Result For Naming Binary Ionic Compounds Ionic Compound Chemistry Worksheets Chemistry Lessons

0 Comments